RE-6H Alkaline Reference Electrode kit
Hg/Hg2O alkaline
reference electrode is made of Mercury and mercury oxide instead of
calomel paste,
and 1 M sodium hydroxide is used as an electrolyte solution. It is
used under high pH environment as reference electrode. Main body is
made of polyacetal resin.
The standard electrode potential of the Hg/Hg2O alkaline electrode in aqueous solution is:
Hg0 + H2O +2e ⇔ Hg + 2OH-
E0 = 0.098 Vs NHE 25°C
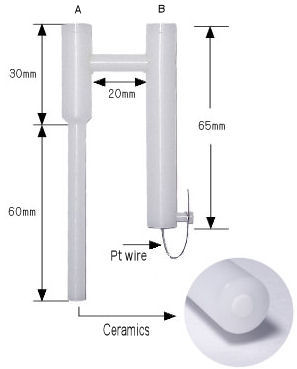
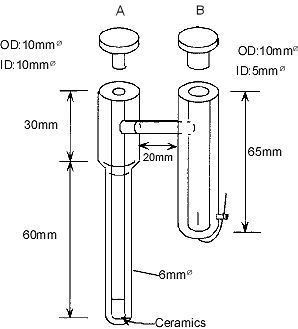
Dimension
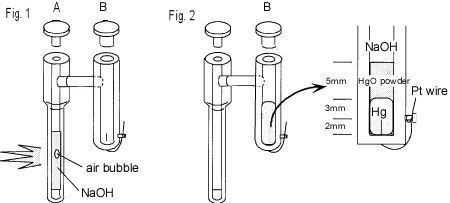
Electrode preparation
The reagents which the customer needs to prepare:
Sodium Hydroxide (NaOH); Mercury (Hg); Mercury (II) Oxide (HgO)
1. 300 µl of 1 M NaOH solution is filled into A tube (shown above)
using syringe and then air bubble
is removed by tapping with finger (Fig. 1).
2. The following reagents are filled based on instructions 1 to 4.
(1) 100 µl of mercury is filled into B tube using syringe
(2) Layer mercury oxide powder onto it up to 5 mm
(3) 1 M NaOH solution is filled until full up the tube, and check
if air bubble is not trapped at the tube
side, so tap it to remove the air bubble
(4) Screw the cap, and check whether leakage is occured or not
3. 1 M NaOH solution is filled up until full up the A tube.
4. Inspect whether air bubble is trapped into the tube under light
illumination.
5. It should be kept at 1 M NaOH solution while it is not used.